NFPA 704 – Chemical Marking and Fire Diamond
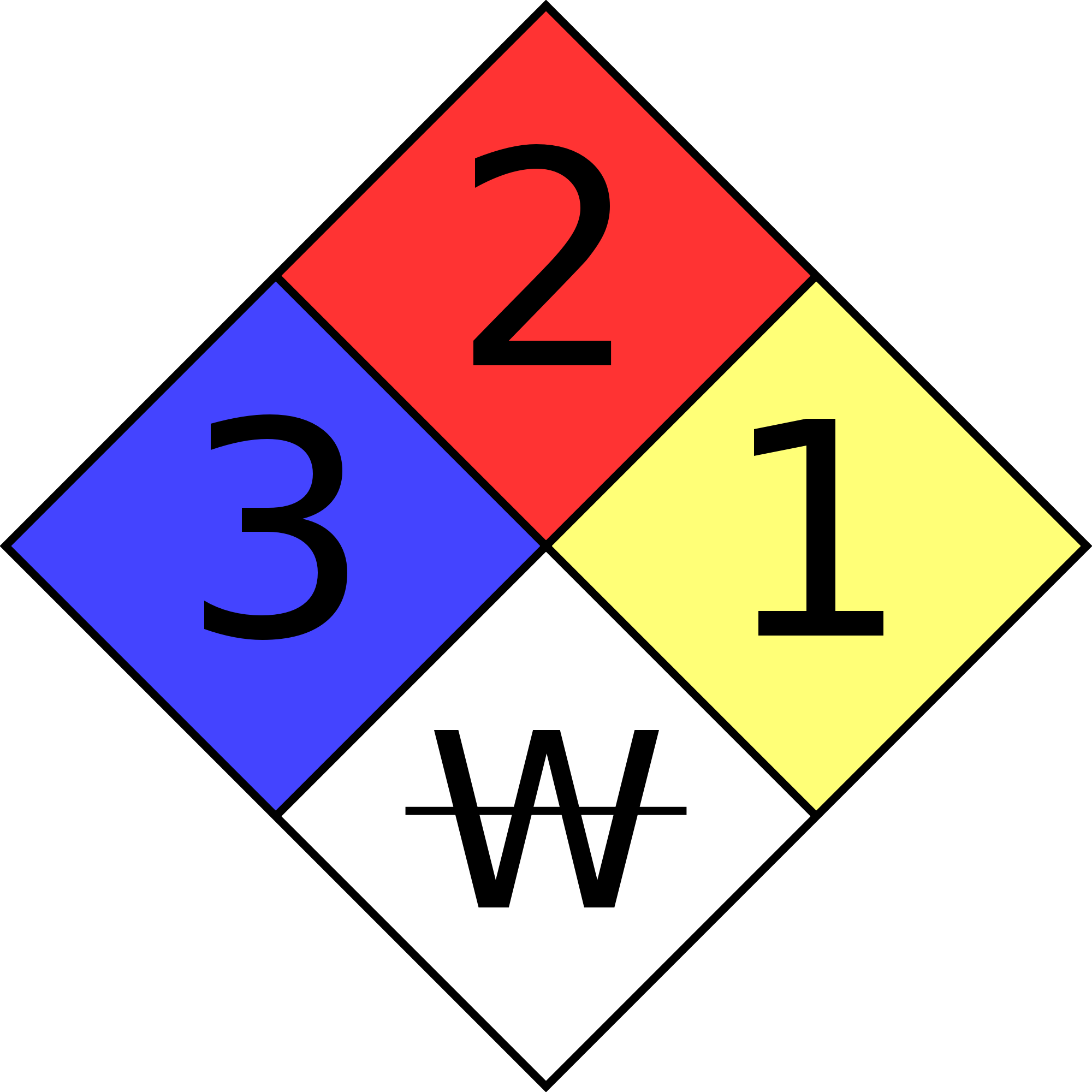
 “NFPA 704: Standard System for the Identification of the Hazards of Materials for Emergency Response” is a standard maintained by the U.S.-based National Fire Protection Association. First “tentatively adopted as a guide” in 1960, and revised several times since then, it defines the colloquial “fire diamond” used by emergency personnel to quickly and easily identify the risks posed by hazardous materials. This helps determine what, if any, special equipment should be used, procedures followed, or precautions taken during the initial stages of an emergency response.
“NFPA 704: Standard System for the Identification of the Hazards of Materials for Emergency Response” is a standard maintained by the U.S.-based National Fire Protection Association. First “tentatively adopted as a guide” in 1960, and revised several times since then, it defines the colloquial “fire diamond” used by emergency personnel to quickly and easily identify the risks posed by hazardous materials. This helps determine what, if any, special equipment should be used, procedures followed, or precautions taken during the initial stages of an emergency response.
Fire Diamond
The four divisions are typically color-coded with:
- Red indicating – Flammability,
- Blue indicating level of Health Hazard,
- Yellow for Chemical Reactivity,
- White containing codes for Special Hazards.
Each of health, flammability and reactivity is rated on a scale from 0 (no hazard) to 4 (severe risk). The latest version of NFPA 704 sections 5, 6, 7 and 8 for the specifications of each classification are listed below. The numeric values in the first column are designated in the standard by “Degree of Hazard” using Arabic numerals (0, 1, 2, 3, 4), not to be confused with other classification systems, such as that in the NFPA 30 Flammable and Combustible Liquids Code, where flammable and combustible liquid categories are designated by “Class”, using Roman numerals (I, II, III).
Flamability
| 0 | Materials that will not burn under typical fire conditions, including intrinsically noncombustible materials such as concrete, stone and sand.
Materials that will not burn in air when exposed to a temperature of 820 °C (1,500 °F) for a period of 5 minutes e.g.: Carbon tetrachloride |
|---|---|
| 1 | Materials that require considerable preheating, under all ambient temperature conditions, before ignition and combustion can occur. Includes some finely divided suspended solids that do not require heating before ignition can occur.
Flash point at or above 93.3 °C (200 °F). e.g.: mineral oil, ammonia |
| 2 | Must be moderately heated or exposed to relatively high ambient temperature before ignition can occur and multiple finely divided suspended solids that do not require heating before ignition can occur.
Flash point between 37.8 and 93.3 °C (100 and 200 °F). e.g. diesel fuel, paper |
| 3 | Liquids and solids (including finely divided suspended solids) that can be ignited under almost all ambient temperature conditions.
Liquids having a flash point below 22.8 °C (73 °F) and having a boiling point at or above 37.8 °C (100 °F) or having a flash point between 22.8 and 37.8 °C (73 and 100 °F). e.g. gasoline, acetone |
| 4 | Will rapidly or completely vaporize at normal atmospheric pressure and temperature, or is readily dispersed in air and will burn readily
Flash point below room temperature at 22.8 °C (73 °F). e.g.: acetylene, propane, liquid hydrogen, pyrophoric substances. |
Health
| 0 | Poses no health hazard, no precautions necessary and would offer no hazard beyond that of ordinary combustible materials
e.g. wood, paper |
|---|---|
| 1 | Exposure would cause irritation with only minor residual injury
e.g. acetone, sodium bromate |
| 2 | Intense or continued but not chronic exposure could cause temporary incapacitation or possible residual injury
e.g. diethyl ether, ammonium phosphate |
| 3 | Short exposure could cause serious temporary or moderate residual injury
e.g. liquid hydrogen, carbon monoxide, calcium hypochlorite, hexafluorosilicic acid |
| 4 | Very short exposure could cause death or major residual injury
e.g. hydrogen cyanide, phosgene, methyl isocyanate, hydrofluoric acid |
Instability/reactivity
| 0 | Normally stable, even under fire exposure conditions, and is not reactive with water
e.g. helium, N2 |
|---|---|
| 1 | Normally stable, but can become unstable at elevated temperatures and pressures
e.g. propene |
| 2 | Undergoes violent chemical change at elevated temperatures and pressures, reacts violently with water, or may form explosive mixtures with water
e.g. white phosphorus, potassium, sodium |
| 3 | Capable of detonation or explosive decomposition but requires a strong initiating source, must be heated under confinement before initiation, reacts explosively with water, or will detonate if severely shocked
e.g. ammonium nitrate, caesium, Armstrong’s mixture |
| 4 | Readily capable of detonation or explosive decomposition at normal temperatures and pressures
e.g. nitroglycerin, chlorine dioxide, nitrogen triiodide, chlorine trifluoride |
Special notice (white)
The white “special notice” area can contain several symbols. The following symbols are defined by the NFPA 704 standard.
| OX | Oxidizer, allows chemicals to burn without an air supply
e.g. potassium perchlorate, ammonium nitrate, hydrogen peroxide |
| ₩ | Reacts with water in an unusual or dangerous manner
e.g. caesium, sodium, sulfuric acid |
| SA | Simple asphyxiant gas.
Specifically limited to the following gases:
|
Non-standard symbols (white)
These hazard codes are not part of the NFPA 704 standard, but are occasionally used in an unofficial manner. The use of non-standard codes may be permitted, required or disallowed by the authority having jurisdiction (e.g. fire department).


